ALPHA experiment takes a closer look at antimatter
The ALPHA collaboration at CERN in Geneva has scored another coup on the antimatter front by performing the first-ever spectroscopic measurements of the internal state of the antihydrogen atom. The results were published in www.nature.com/nature/journal/v548/n7665/pdf/nature23446.pdf">https://www.nature.com/nature/journal/v548/n7665/pdf/nature23446.pdf">Nature earlier this year in August. Their measurement showed the hyperfine splitting of antihydrogen is the same as that of hydrogen to four parts in 10,000.
Ordinary hydrogen atoms are the most plentiful in the universe, and also the simplest – so simple, in fact, that some of the most fundamental physical constants have been discovered by measuring the tiny energy shifts resulting from the magnetic and electric interactions of hydrogen’s proton nucleus with its single orbiting electron.
Antihydrogen, on the other hand, is rare, with single positrons orbiting single antiprotons – difficult to make, and even more difficult to hold onto. Indeed antihydrogen had never been trapped until ALPHA succeeded in doing so in 2010.
ALPHA researchers created and captured hundreds of antihydrogen atoms in a magnetic bottle, then probed their internal states by bathing them in microwave radiation that flipped the spins of the positrons, causing the immediate ejection of the atoms from the magnetic trap and their annihilation on the trap wall. Antihydrogen should behave the same way as hydrogen, and the frequency of the microwave radiation required to flip its spins thus provides a direct measure of the difference in energy between the two hyperfine states of antihydrogen.
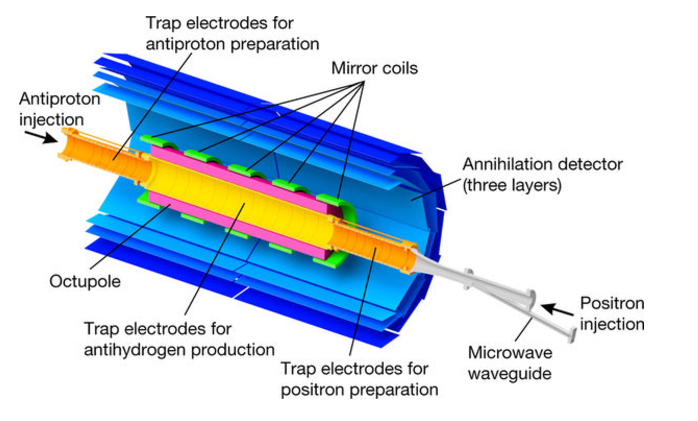
Fig.1 The ALPHA central apparatus: a cut-away schematic of the antihydrogen production and trapping region of ALPHA-2 is shown ((Image Credits: Nature).
The current experiment is measuring that several-million-times-smaller hyperfine splitting, using basically the same principle: they collect a bunch of antihydrogen in a magnetic trap, hit them with light of the appropriate frequency, and see how many atoms are left. If the frequency of the light they're hitting the anti-atoms with matches the transition frequency, the resulting state change causes atoms to fall out of their trap and annihilate with ordinary matter in the walls, which they can detect.
The process is complicated by the fact that their trapped atoms are held in a huge magnetic field, which shifts the energy of the electron orbits. They're rescued by a quirk of atomic structure, though, which is that in the high-field limit, the states split into two groups of two, giving two transition frequencies that differ by exactly the hyperfine splitting. This lets them do something that looks a lot more like ordinary spectroscopy. In the December paper, they fixed the laser at the frequency for ordinary hydrogen and confirmed that it caused losses of antihydrogen. In this paper, they vary the microwave frequency to find the maximum loss for one of the transitions, then increase the frequency by approximately the hyperfine splitting for hydrogen, and repeat the process to find the maximum for the other.
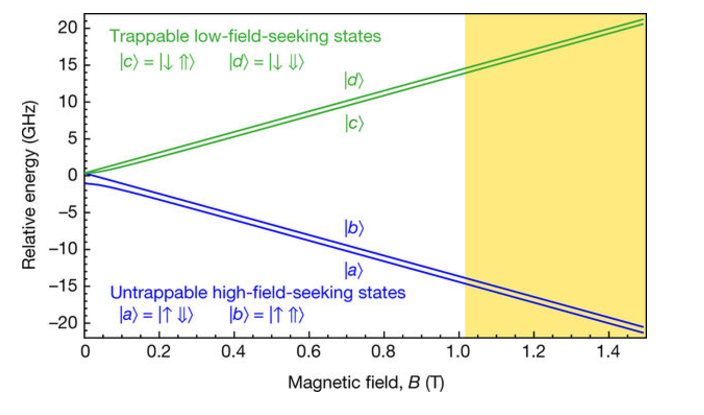
Figure 2: Ground-state hyperfine energy levels. The energy levels are calculated assuming they are identical to those of hydrogen. The ket notation indicates the positron spin (left; ↓ or ↑) and antiproton spin (right; ⇓ or ⇑) states in the high-field limit. The shaded region illustrates part of the range of fields in the ALPHA-2 antihydrogen trap, with the minimum at 1.03 T.
The experiment involves slowly scanning the microwave frequency and measuring the number of annihilation events. The result is two peaks, with the hyperfine splitting corresponding to the energy difference between the peaks.
The team's measured value for antihydrogen hyperfine splitting (expressed in terms of photon frequency) is 1420±0.5 MHz, which agrees with the measured value for hydrogen to four parts in 10,000.
The hyperfine splitting might be a good place to look for differences between hydrogen and anti-hydrogen. The hyperfine interaction is between the spin of the proton and the spin of the electron, which means it's both very weak (since the magnetic field generated by either is tiny) and very short-range (because it's a dipole interaction, rather than the direct charge-charge interaction). Much of the shift happens thanks to interactions when the electron is inside the nucleus, and that's exactly the sort of scenario where you'd expect exotic physics to show up.
“Spectroscopy is a very important tool in all areas of physics. We are now entering a new era as we extend spectroscopy to antimatter,” said Jeffrey Hangst, Spokesperson for the ALPHA experiment. “With our unique techniques, we are now able to observe the detailed structure of antimatter atoms in hours rather than weeks, something we could not even imagine a few years ago.”
With this new result, the ALPHA collaboration has clearly demonstrated the maturity of its techniques for probing the properties of antimatter atoms.
The rapid progress of CERN’s experiments at the unique Antiproton Decelerator facility is very promising for even more precise measurements to be carried out in the near future.
