ISOLDE sheds light to the nature of the proposed magic number N = 32
The most intuitive properties of the nucleus are its mass and size. Both properties can be precisely studied with dedicated experiments at ISOLDE. Most of the nuclei are deformed, i.e. shaped like a rugby ball or like a pancake, only a few are spherical and present extra degrees of stability. These nuclei are more difficult to excite, they contain a certain number of neutrons and protons called "magic". This extra stability is similar to the shell closing of valence electrons responsible of the lack of reactivity of noble gases. As for the electrons in atoms, the protons and neutrons in the atomic nucleus occupy quantum levels grouped in shells, separated by energy gaps. The magic numbers appear in nuclei with protons or neutrons completely filling a shell.
The large energy gap to the next shell makes these nuclei difficult to excite. This discovery, made separately by M. Goeppert-Mayer and J. D. Jensen, merited the Noble Prize in 1963. The magic neutron and proton numbers: 2, 8, 20, 28, 50, 82 work well for nuclei close to the stable ones. Far from stability the nuclei have an unbalanced composition of neutrons and protons. The neutron-neutron and neutron-proton residual interactions change dramatically, giving rise to new “magic” numbers for these exotic nuclei. A fundamental understanding of how the nuclear structure evolves in unstable neutron-rich nuclei, and how they impact the charge radius, is one of the main challenges of modern nuclear physics.
The figure represents the chain of calcium isotopes studied in our work. The “doubly-magic” isotopes with mass numbers 40 (40Ca) and 48 (48Ca) display similar charge radii, while the measured value for the suggested "doubly-magic" 52Ca is larger than expected.
The calcium isotopic chain (Z=20, magic proton number) is a unique nuclear system to study how protons and neutrons interact inside the atomic nucleus: two of its stable isotopes are magic in both their proton and neutron number (40Ca with N=20 and 48Ca with N=28). In addition, experimental evidence of doubly-magic features in another short lived calcium isotope, 52Ca, has been obtained at ISOLDE based on mass measurements [Wienholtz et al, Nature 498 (2013) 346].
Although the average distance between the electrons and the nucleus in an atom is about 5000 times larger than the nuclear radius, the size of the nuclear charge distribution is manifested as a perturbation of the atomic energy levels. A change in the nuclear size between two isotopes gives rise to a shift of the atomic hyperfine structure (hfs) levels. This shift between two isotopes, one million times smaller than the absolute transition frequency, commonly known as the isotope shift, includes a part that is proportional to the change in the nuclear mean square charge radii. A measurement of such a tiny change is only possible by using ultra-high resolution techniques. The collinear laser spectroscopy technique developed at ISOLDE has been established as a unique method to reach such high resolution and has been applied with different detection schemes to study a variety of nuclear chains.
Despite an excess of eight neutrons, 48Ca exhibits the striking feature that it has identical charge radius to 40Ca. A fundamental explanation of this anomalous feature has been a longstanding problem for nuclear theory. To determine the radius beyond 48Ca was therefore crucial both from an experimental and theoretical point of view especially after the recently suggested magic character of 52Ca. The measurements were performed by using high-resolution bunched-beam collinear laser spectroscopy using the COLLAPS installation at ISOLDE. The charge radii for 40-52Ca isotopes were obtained from the measured optical isotope shifts. With a production yield of only a few hundred ions per second, the measurement of 52Ca represents one of the highest sensitivities ever reached by using fluorescence detection techniques. The isotope shifts were extracted from the fit of the hfs experimental spectra. The resulting charge radius of 52Ca is found to be much larger than expected for a doubly-magic nucleus and significantly exceeds the theoretical predictions.
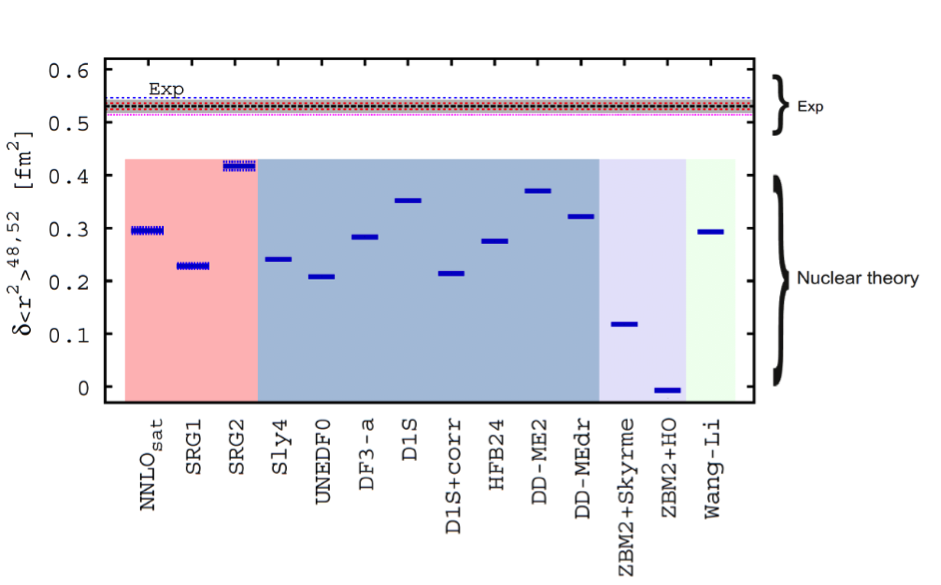
The figure shows the experimental root mean square charge radius in 52Ca relative to that in48Ca. Experimental uncertainties are represented by the horizontal dotted lines (statistical) and the grey shaded band (systematic). The experimental results are compared to different theoretical predictions representative of modern approaches to charge radii (horizontal lines).
The large and unexpected increase of the size of the neutron-rich calcium isotopes beyond N = 28 challenges the doubly-magic nature of 52Ca and opens new intriguing questions on the evolution of nuclear sizes away from stability, which are of importance for our understanding of neutron-rich atomic nuclei.
